HLB System in Silicone Emulsions
The Hydrophilic-lipophilic balance of a surfactant is a measure of the degree to which it is hydrophilic or lipophilic, determined by calculating values for the different regions of the molecule.
HLB = 20 * Mh/M
Briefly, the HLB System enables you to assign a number to the ingredient or combination of ingredients you want to emulsify, and then to choose an emulsifier or blend of emulsifiers having this same number. An emulsion is a system of two (or more) immiscible materials (usually liquids) in which one material (the dispersed/ internal phase) is suspended or dispersed throughout another material (the continuous/external phase) in separate droplets. Most emulsions fall into two different classes, oil in water emulsions and water in oil emulsions. In oil in water emulsions, we have hundreds of tiny oil droplets surrounded by water.
![]()
In the water in oil emulsions, we have the opposite situation. We have hundreds of water droplets surrounded by oil. All emulsifiers have two parts; like a bar magnet. A bar magnet has a north pole and a south pole. Nonionic emulsifiers also have two poles or parts. An emulsifier molecule has one part that loves water and one part that loves oil. The water-loving part is called hydrophilic; “hydro” means water and “philic” means to love or like. The other part of the emulsifier molecule is lipophilic; if “lipo-” means fat or oil, then lipophilic means oil-loving. Therefore, an emulsifier has a hydrophilic part and a lipophilic part. The balance of these two portions of the emulsifier gives us the Hydrophile-Lipophile Balance (HLB). The HLB of emulsifiers can be calculated or determined through trial and error. According to the HLB System, all fats and oils have a Required HLB. For example, if I wanted to emulsify Soybean Oil, which has a required HLB of 7, I would need to use an emulsifier or blend of emulsifiers that had a HLB of 7±1. Before we can select our emulsifiers that we will need, we must know the required HLB of our oil phase.

Through long experience in using the HLB System, we have found that all oils, waxes and other materials likely to be incorporated into emulsions have an individual “Required HLB.” For instance, the required HLB for a fluid O/W, emulsion of paraffin is 10. This means that an emulsifier, or blend of emulsifiers, having an HLB of 10 will make a more stable fluid O/W paraffin emulsion than emulsifiers of any other HLB value. It does not mean that every emulsifier or blend having an HLB of 10 will “work” - you might have an “HLB 10” emulsifier of the “wrong” chemical family (wrong for this purpose, at least). However, you can be assured that when you are working with any certain family of emulsifiers, you will obtain optimum results more quickly if you work in the area of HLB 10, say ± 1. You would be wasting time to try emulsifier blends atHLB 8 or 13, for example, unless you might happen to be looking for a particular quality other than stability in your emulsion. Do not make the mistake of assuming, from this preliminary working data, that you should immediately try all single emulsifiers in the catalog that have an HLB of 10 for your paraffin emulsion.
Remember, you can blend emulsifiers to make any HLB you want, and blends usually work best. Suppose you are making an O/W emulsion textile lubricant. The product might be 30% mineral spirits, 50% cottonseed oil and 20% chlorinated paraffin to be emulsified in water. The required HLB of the combination can be calculated as follows:
Mineral Spirits ..........30% X Req. HLB 14 = 4.2
Cottonseed Oil ..........50% X Req. HLB 6 = 3.0
Chlorinated Paraffin . . 20% X Req. HLB 14 = 2.8
Estimated HLB for emulsifier system ..........10.0
For the control of your emulsion after emulsification, there are different methods. For microemulsions, for example, one response that can be measured is particle size. The lower the particle size, the clearer the emulsion appearance. A typical curve generated by the HLB method for a microemulsion of 15% amino-functional silicone oil ( TSF 4708 Momentive Performance Materials ) in water is:
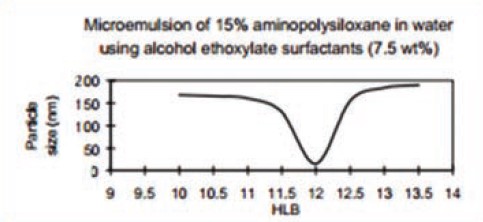
The microemulsion of 15% amino-functional silicone oil in water using alcohol ethoxylated surfactants (7.5 wt%) This indicates optimum appearance is achieved at HLB 11.5-> 12.5.
This method helps to narrow in on the required HLB value of the system being studied. In general, a mixture of surfactants (of high & low HLB) gives a more stable emulsion because:
(a) The spread of size of the lipophiles and/or hydrophiles allows more efficient packing at the oil/water interface
(b) The interface can be supplied with surfactants from both the oil (more lipophilic surfactant) and water (more hydrophilic surfactant) phases in more equal proportions rather than predominantly from one side, as would be the case with a single surfactant.
(c) A mixture of surfactants gives larger polydispersity to more effectively match the polydispersity of the oil phase.
The HLB method, however, is not predictive of emulsion stability and therefore different surfactant types should now be tested to achieve optimum emulsion stability e.g. linear/branched alcohols. In general, the HLB of oilin- water polysiloxane emulsions falls in the range 10 to 14. In general, non-ionic alcohol ethoxylates are employed as surfactants i.e. RO(CH2CH2O)xH , where R is an alkyl group e.g. C9H19/C11H23, i-C13H27 x, is any positive integer, in order to allow compatibility with ionic auxiliaries (e.g. optical brighteners).
For your preliminary tests, to determine your required HLB, select any matched pair of SPAN and TWEEN emulsifiers, i.e. SPAN 20 with TWEEN 20 or SPAN 60 with TWEEN 60. This will give you two emulsifiers of the same chemical class, one lipophilic (oil-loving), the other hydrophilic (water-loving). For example, the “20” SPAN-TWEEN emulsifiers are both laurate esters; the “40”s are palmitate esters; the “60”s are stearates, and the “80”s oleates. The SPAN emulsifiers are lipophilic, the TWEEN products hydrophilic. This is only a trial run, so you do not care at this point whether the emulsifiers you select are perfect
for your purpose or not. Suppose you happen to have some SPAN 60 and TWEEN 60 on your lab shelf.
You can use these for your trials. As a start, make up small batches of seven emulsifier combinations, ranging in HLB from straight SPAN 60 (HLB = 4.7) to a straight TWEEN 60 (HLB = 14.9),* as follows:
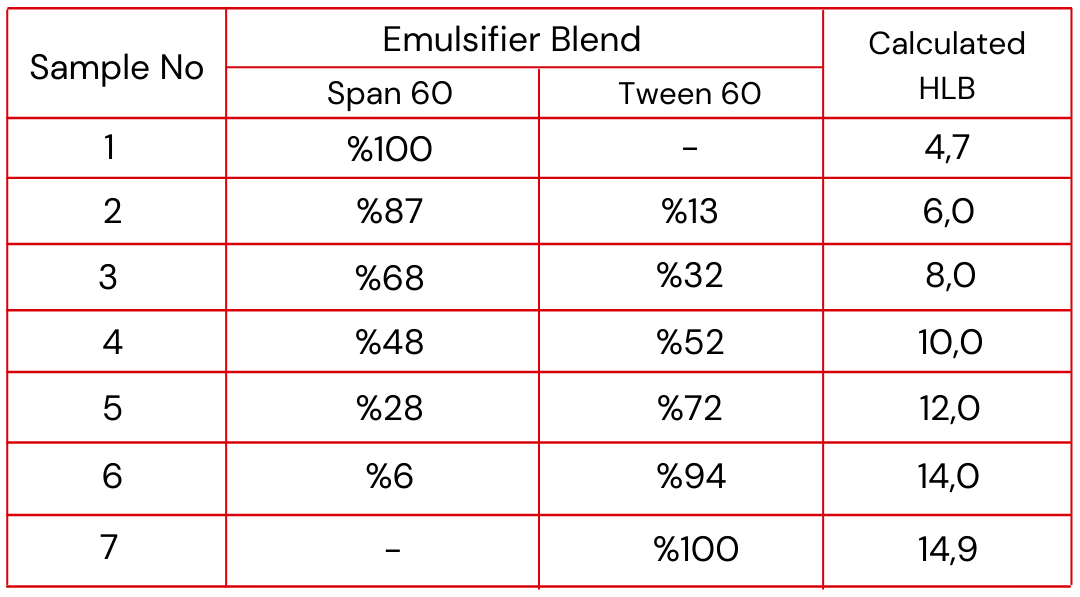
Now, make seven test emulsions, using one of the above emulsifier samples in each. Use an excess of emulsifier (say 10-20% of the weight of your oil phase), and dissolve or intimately disperse the emulsifier into the oil phase, melting ingredients together if necessary. While simple mixing of your ingredients and emulsifiers will probably be sufficient at this point in your testing, it is important that you use preparation methods as nearly identical as possible for each of your seven emulsions, simulating your own plant methods.
Using the appropriate method or methods for comparison and evaluation of your product, you will probably notice fairly quickly that one or another of these emulsifier combinations will give you a better emulsion than the other six, even though not necessarily a very good one. If all the emulsions seem fairly good, with not much noticeable difference, then repeat the seven tests, using less emulsifier. Conversely, if all the emulsions are poor and show no great difference, repeat the tests but use higher emulsifier content.
Step by Step Outline of HLB System for Selecting Emulsifiers;
Step One Determine the “Required HLB” for the oil or other ingredients you wish to emulsify.
Step Two Try different chemical types of emulsifier blends, adjusted close to the “Required HLB” you found in Step One. You save time because you do not need to try any other blends than those at your predetermined “Required HLB.”
Step Three If your emulsion experience indicates a trial of other chemical types of emulsifiers, you can still save much time by determining the HLB of these emulsifiers. If one specific familiar emulsifier does not have your “Required HLB” (as determined in Step One), then you should blend it with another emulsifier to obtain this “Required HLB” for optimum results.
References:
Griffin, W. C. Classification of Surface Active Agents by HLB. J. Soc. Cosmet. Chem. 1949, 1, 311-326. Griffin, W. C. Calculation of HLB Values of Nonionic Surfactants,
J. Soc. Cosmet. Chem. 1954, 5, 249-256 Pasquali, R. C; Taurozzi, M. P; Bregni, C; “Some Considerations about the Hydrophilic-Lipophilic Balance System”, Intl.
J. Pharmaceutics 356 (2008) 44-5. Factors Affecting Emulsion Stability, and the HLB concept J
Boyd, C Parkinson1, P Sherman1.